Many gardeners do not realise the large effects that pH has on nutrient availability for their plants. An acid soil, that is a soil with a low pH, or a soil with a very high pH, alkaline, is effectively deficient in nutrients even when nutrients have been added to the soil.
Understanding the pH scale
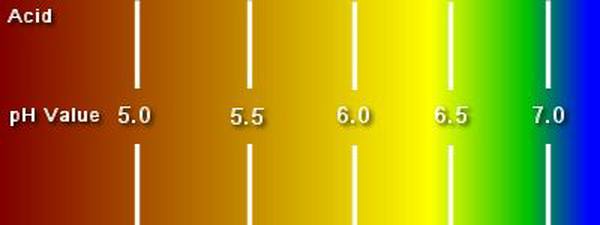
When measuring soil acidity it is important to understand that the pH scale is logarithmic. That a pH of 4 is ten times more acidic than a pH of 5 and 100 times more acidic than a pH of 6.
The ideal pH for most vegetables lies between 6.5 and 7.0. Soil outside of these limits will limit growth and reduce the health of crops making them more vulnerable to weather extremes, diseases and pests.
In the UK soil pH will have a natural range between 5.00 and 7.50. Most soils tend to be acid but soils lying on a chalky base will tend to be alkaline.
Unless the soil is lying on a chalky base, if it hasn’t been limed for years it is most likely acid. Most garden and allotment soils will have a pH around 5.5, ten times more acid than ideal. The addition of fertilisers and manures and the natural actions of microbes in the soil acidifies soil.
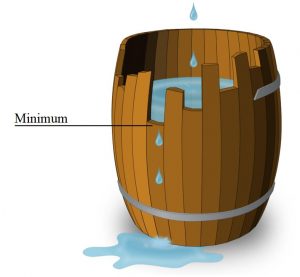
Leibig’s Law of the Minimum
Another concept or principle that should be kept in mind is the ‘Leibig’s Law of the Minimum’ which was stated and explained by Justus von Leibig, a German chemist in the 1800s.
The law was originally applied to plant growth, where it was found that increasing the amount of plentiful nutrients did not increase plant growth. Only by increasing the amount of the limiting nutrient (the one most scarce in relation to need) was the growth of a plant improved.
In other words, “The availability of the most abundant nutrient in the soil is only as good as the availability of the least abundant nutrient in the soil.”
Most often this is illustrated by a barrel with staves of different lengths. No matter how much water is poured into the barrel, the level cannot rise above that of the lowest stave.
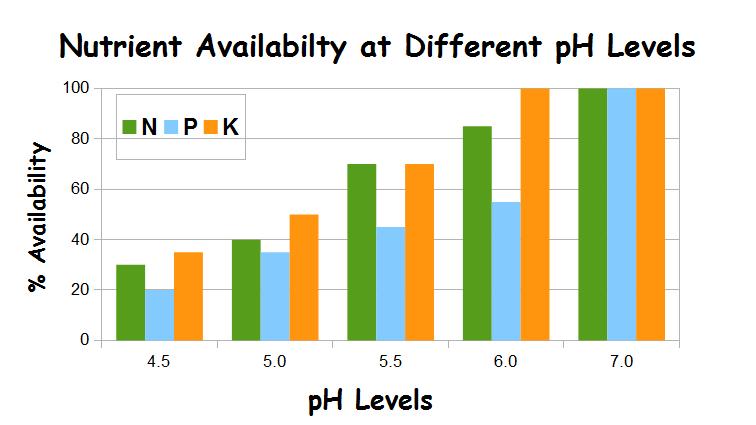
Main Nutrients Affected by pH Level
The availability of the ‘big 3’ nutrients: N – nitrogen, P – phosphorus, K – potassium or potash, is affected by the pH level. In a very acid soil of pH 5.0 only 40% of the nitrogen is available, 35% of the phosphorus and 50% of the potassium. Increasing to an average plot’s pH 5.5 takes the nitrogen and potassium up to 70% availability but the phosphorus is still only at 45% availability. It’s not until the pH hits 6.5 that all the big 3 nutrients are fully available to fuel the plants.
Effect of pH level on micro-nutrient availability
Plants require other nutrients – generally referred to as micro nutrients – and these are adversely affected by extremes of acidity. A pH below 6.0 will have a noticeable effect on calcium and magnesium availability.
Above pH 7.0 Iron and manganese availability declines and in very alkaline soils with a pH of 7.5 boron, copper and zinc availability reduces dramatically.
Correcting the pH will have the same – or better – effect as applying nutrients in whatever form; compost, manure or fertiliser.